Blood-culture contamination remains a problematic cause of false positives and diagnostic errors for many clinical laboratories.
Blood-culture contamination remains a problematic cause of false positives and diagnostic errors for many clinical laboratories. According to the College of American Pathologists (CAP), a blood culture is considered contaminated if one or more of the following organisms are identified in only one culture of a series of specimens in a 24-hour period:1
- Coagulase-negative Staphylococcus species
- Propionibacterium acnes
- Micrococcus species
- Alpha-hemolytic viridans group streptococci
- Corynebacterium species (diphtheroids)
- Bacillus species
This contamination determination does not take into account any other culture results; only when the above-mentioned organisms are found in blood cultures across multiple draws (from a single patient) in a 24-hour period are they considered pathogenic.
Set a Stringent Standard
CAP has established a blood-culture contamination rate threshold of 3% for corrective action. However, laboratory leadership at Mayo Clinic Health System – Franciscan Healthcare (MCHS-FH) in La Crosse, Wisconsin, felt the lab could, and should, establish a more stringent threshold than 3% after accounting for the cost-of-poor-quality (COPQ) impacts on patients. Multiple studies have estimated the cost of an inpatient stay stemming from a contaminated blood culture to be between $2,889 and $8,720, on average, with increased lengths of stay estimated at up to 3.3 days.2 This does not include the additional cost to labs, which can approach $1,000 per contaminated culture.
At MCHS-FH, each positive culture is treated as a true positive until the organism identification (ID) is completed. The testing to determine contamination is based on the initial organism ID by routine (traditional) culture and the number of positive cultures within a 24-hour period.
Current literature states that the leading cause of contamination in blood samples is improper site preparation to eliminate skin flora—a process well within the phlebotomist’s control. With this in mind, beginning in October 2014, MCHS-FH leadership set a project goal of a less than 2% contamination rate for its laboratory. The subsequent two months were spent observing existing phlebotomy practices—specifically sample collecting and any actions that could hinder improvement. Inconsistencies were noted among phlebotomists as to how thoroughly to clean the draw site with antiseptic to render it truly sterilized.
Raising Awareness
The laboratory’s contamination rate during the month of October 2014 was 2.9% with 15 contaminated cultures (4 of the previous 8 months likewise experienced contamination rates above 2%). The COPQ impact on patients for the month of October 2014 was estimated to be between $43,335 and $130,800, along with 49.5 additional days of hospitalization. Contributing to this extra expense is logical clinician practice—rather than jeopardize a patient’s outcome, physicians treat each positive culture as pathogenic until contamination is confirmed. Thus, any treatment adds cost.
To achieve and maintain the less than 2% contamination goal, lab leadership recognized its necessary role in engaging frontline staff to raise awareness of COPQ for process and outcome defects. By employing Lean methodologies—founded on respect for people and the provision of tools to assist leaders in coaching staff in problem-solving to improve quality—this project addressed contaminated blood cultures by incorporating change-management techniques intended to motivate staff to improve collection and training processes, thereby ensuring patient safety.
Typically, the collection and review of practice data alone do not drive behavioral change. Thus, lab leadership began the improvement journey by discussing with staff the additional costs and overnight hospital stays patients endure due to contaminated cultures. In addition, unnecessary increases in patients’ length-of-stay (LOS) were considered a contributing factor to the hospital having to divert certain patients to another hospital for care. Most important, extended hospital stays also increase a patient’s risk of hospital-acquired infections.3
Once awareness was raised on how contaminated cultures negatively impact patients, staff phlebotomists were inspired to improve the lab’s contamination rates from a compassionate, emotional perspective.
Improved Blood Culture Protocols
After observing and noting the practices of senior phlebotomists who had produced no contaminated cultures during the observation period, leadership implemented those techniques, coupled with best practices indicated in the literature, as the standard operating procedures for phlebotomy. Exemplary staffers also were asked to help train coworkers on contamination countermeasures that include:
- Ensuring the collection site is sterile by performing a friction scrub of 30 to 60 seconds to expose underlying bacteria to the antiseptic that follows.
- After the friction scrub, apply antiseptic in a 2– to 3-inch concentric circle from collection site outward.
- Let the site air dry for at least 30 seconds (no waving of the hand or excessive movement to expedite the drying process).
- No re-palpation or touching of the cleansed site to re-find a vein (surgical gloves are not sterile, and fingertip sanitation is not advised).
- Establish and be certain of the draw site before cleaning.
- Require venipuncture and avoid line draws.
Over the last two decades, a national trend contributing to higher blood contamination rates is the widespread delegation of blood-culture collection activities to non-phlebotomists (eg, nurses) who tend to have less extensive training, experience, and knowledge about the procedure than a phlebotomist.4 At MCHS-FH, non-phlebotomists (who perform about 5% to 10% of blood cultures) are trained by senior lab technicians and senior phlebotomists before being permitted to perform the procedure.
Visual Management
Group Performance Charts
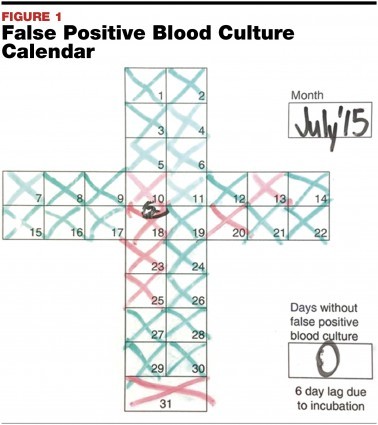
The use of visual aids also proved valuable in promoting increased employee engagement. With this leadership concept in mind—You cannot improve on what you cannot see or measure—MCHS-FH instituted the display of daily contamination results via a safety cross chart (albeit with a six-day lag to account for the incubation period of blood cultures). Leadership receives the final results each morning and updates the safety chart with a red X indicating a contamination and a green X when there is none. This real time monitoring of results has promoted an infectious interest among staff (see FIGURE 1).
Whereas the safety cross chart uses a simple X method to illustrate contamination incidence on a daily basis, an additional control chart was implemented to track contamination rates over broader time periods. This chart offers monthly stats of total cultures drawn with any applicable contamination percentages, then further displays a graph of contamination rates across the year.
Collection Cards
A collection card was implemented as a visual tool for phlebotomists to document methods and issues related to each collection. The lab adopted the card idea from its sister site, Mayo Clinic in Rochester, Minnesota. The cards remind phlebotomists that they are performing a blood culture draw—a process that necessitates a specific technique and requires special care compared to other types of draws. Each card includes the chronological steps for prepping the draw site, which must be checked off by the phlebotomist as the procedure is performed. The phlebotomist then writes down the type of draw and amount of blood taken (10 ml is minimum for a culture sample).
Collection cards provide phlebotomists with a task to perform while the iodine antiseptic dries and encourages diligence in collection site cleaning. This collection card then accompanies the related blood culture to the laboratory to inform staff of all relevant information.
A Fishbone Diagram
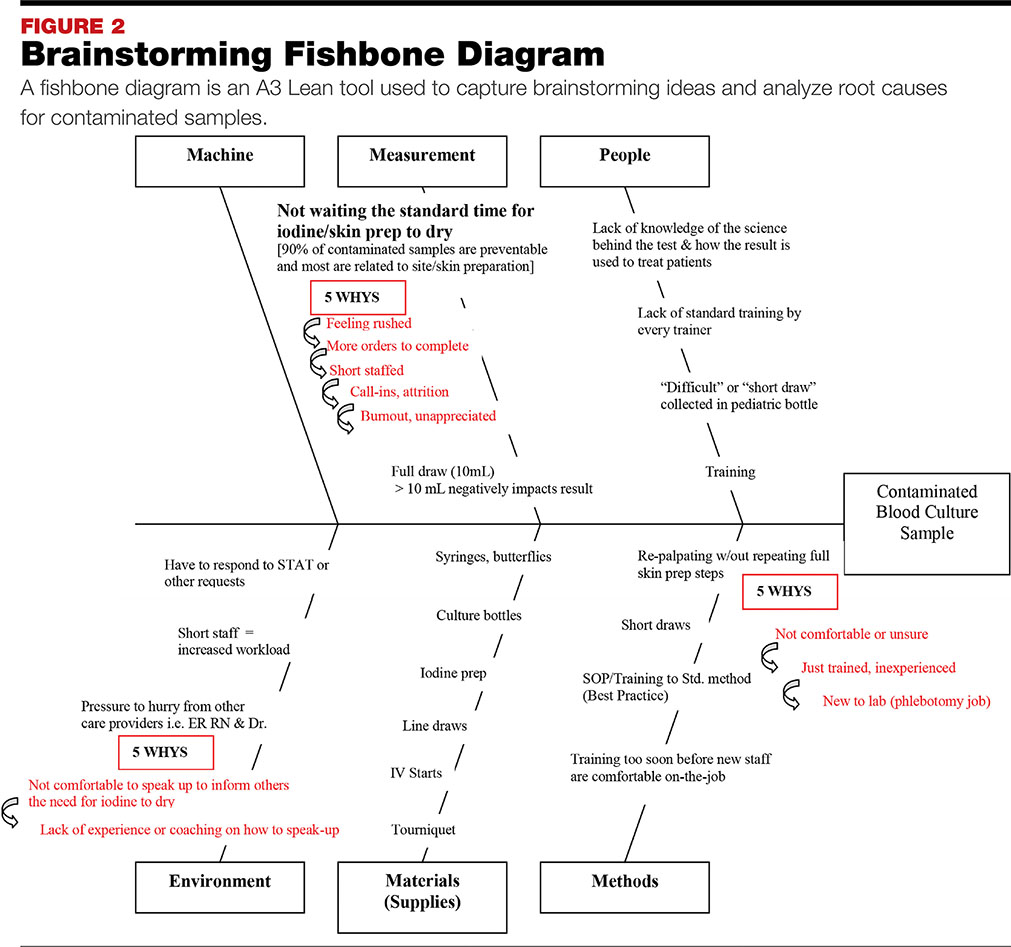
Another valuable implementation was the A3 Lean tool, which includes the use of a fishbone diagram to capture brainstorming and analyze root causes for contaminated samples (see FIGURE 2). The A3 is a problem-solving tool that can be used to help understand process problems, such as those encountered during blood culture collection. It aids in identifying, analyzing, and responding to root causes, and allows for the documentation of goals and the development of action plans for process and quality improvements.
Training New Staff
A key intervention in phlebotomy education involved deferring the hands-on training of new staff on blood-culture collection by at least three months. The three-month delay allowed new employees to become comfortable with the basic skills of a phlebotomist first—especially acquiring the touch needed to locate a patient’s vein—and work toward gaining the confidence to perform a blood-culture collection. In other words, leadership did not want to put pressure on new staff to immediately learn a complex procedure or worry about contamination. Furthermore, many new phlebotomists start out on the night shift when fewer senior staff members are available to help.
This delay was not without its challenges, as it did contribute to resentment and guilt among staff. Some senior phlebotomists complained that they had to perform blood cultures as soon as they were hired and resented the extra work, whereas new staffers expressed guilt over placing the burden for complex collections on their senior colleagues and felt that they were not able to fully contribute during the training delay.
Fortunately, these issues began to be mitigated when contamination rates fell significantly, a result that correlated directly with the delayed training protocol. In 2014, the laboratory had a total of 93 contaminated blood cultures out of 5,784 collected, equaling a contamination rate of 1.6%. Over the course of 2015, there were a total of 79 contaminated cultures out of 5,863 collected, further reducing the contamination rate down to 1.3%. (Anything below 1% is considered improbable due to uncontrollable factors such as transient bacteremia.) High-end COPQ to patients over these two years was reduced by an estimated $122,080.
Team Building
The first time the laboratory hit 30 days with no blood culture contaminations, the occasion was celebrated as a landmark accomplishment. Subsequent 30-day stretches with no contaminated blood cultures continue to be celebrated as such observances boost morale and inspire staff members to remain vigilant in sustaining success.
No individuals are singled out during these celebrations in order to maintain a team effort—the contamination rate is considered a team rate. During such celebrations, new staff members who are still in the delayed training period tend to be hesitant to partake. Many feel as though they do not deserve to join in because they are not yet performing blood cultures. However, they are taught that by waiting to be properly trained, they too are contributing to reduced contaminations.
Daily, face-to-face team huddles also help keep the team motivated to stay engaged in this ongoing process-improvement project. Huddles are important to change management and help establish a culture where problems are no longer considered negative, but as opportunities for positive change. To that end, leadership uses a huddle board to measure improvements in three specific systems: visual management, problem solving, and communication. The huddle board facilitates the tracking of quality and safety issues such as blood-culture contamination rates.
Conclusion
To ensure ongoing success, a Lessons Learned (LL) methodology was employed that included offering all staff the opportunity to document what went well and what could have been improved. The LL is referenced at the beginning of quality-improvement projects to aid in developing a risk-management plan to address performance issues. The benefits of engaging and involving frontline staff members in this continuous-improvement project were achieved by first expressing appreciation for their dedication in providing exceptional service with great care, respect, and compassion.
MCHS-FH leadership is currently sharing its knowledge with other Mayo sites across Wisconsin and Minnesota so that sister laboratories can improve their blood-culture contamination rates through these methods. Sharing effective contamination countermeasures is of utmost importance in creating and maintaining efficient positive change. Such changes to reduce culture contamination can be simple, and there are many papers showing the effects and repercussions of contaminated cultures. It is important that this information and best practices are clearly shared between hospital leadership and laboratory staff who are directly performing the work.
References
- College of American Pathologists. Blood Culture Contamination. Quality Management. Accessed 2/15/17. https://estore.cap.org/OA_HTML/ibeCCtpItmDspRte.jsp?section=10231&item=345861&sitex=10020:22372:US
- Paxton A. Contaminated blood cultures: Taking bold steps to lower rates. CAP Today. Accessed 2/15/17. www.captodayonline.com/Archives/0412/0412c_contaminated.html
- Krell RW, Girotti ME, Dimick JB. Extended length of stay after surgery: Complications, inefficient practice, or sick patients? JAMA Surg. 2014;149(8):815-20.
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802.
Client
MedicalLab Management Magazine
Date
March 2017
Co-Authors
Dana J. Sorenson
Heidi Miksanek
Matthew Tradewell, CT(ASCP), MBA
Kristin Hagen